The formation of coordinate complexes is a fundamental concept in chemistry, particularly in the field of coordination chemistry. It involves the bonding of a ligand to a central atom or ion, where the ligand donates one or more electron pairs to the central atom. This process is made possible by the presence of electron donating groups, which play a crucial role in coordinating with the central atom and stabilizing the resulting complex.
In this article, we will explore the various aspects of electron donating groups and their importance in forming coordinate complexes. We will discuss the definition, types, and examples of these groups, as well as how they contribute to the overall stability and reactivity of the complexes. So, let’s dive into the world of coordination chemistry and unravel the mystery behind electron donating groups!
1. Understanding Electron Donating Groups
Electron donating groups, also known as Lewis bases, are atoms or functional groups that have an excess of electron density and can donate lone pairs of electrons to a central atom or ion. This donation creates a coordinate bond between the ligand and the central atom, thereby forming a coordinate complex.
Types of Electron Donating Groups
There are several types of electron donating groups, each with its own characteristics and reactivity. Some common examples include:
- Alkyl Groups: These are groups containing only carbon and hydrogen atoms, such as methyl, ethyl, and propyl groups. They are weakly electron donating due to the presence of a polar carbon-hydrogen bond.
- Oxygen Donors: These groups contain oxygen atoms and can donate lone pairs of electrons to the central atom. Examples include alcohols, ethers, and carboxylic acids.
- Nitrogen Donors: Groups containing nitrogen atoms, such as amines and amides, are strong electron donors and form stable coordinate complexes.
- Phosphorus Donors: Phosphines, which contain a phosphorus atom, are also strong electron donors and are widely used in coordination chemistry.
How do Electron Donating Groups Contribute to Coordinate Complex Formation?
The presence of electron donating groups is essential for the formation of coordinate complexes. These groups donate their lone pairs of electrons to the central atom, forming a coordination bond. This donation increases the electron density around the central atom, making it more electrophilic and facilitating its bonding with the ligand.
Moreover, electron donating groups also help in stabilizing the resulting complex by providing additional bonding interactions. These groups can also influence the reactivity and selectivity of the complex towards various reactions, making them valuable tools in synthetic chemistry.
2. Examples of Coordinate Complexes Formed by Electron Donating Groups
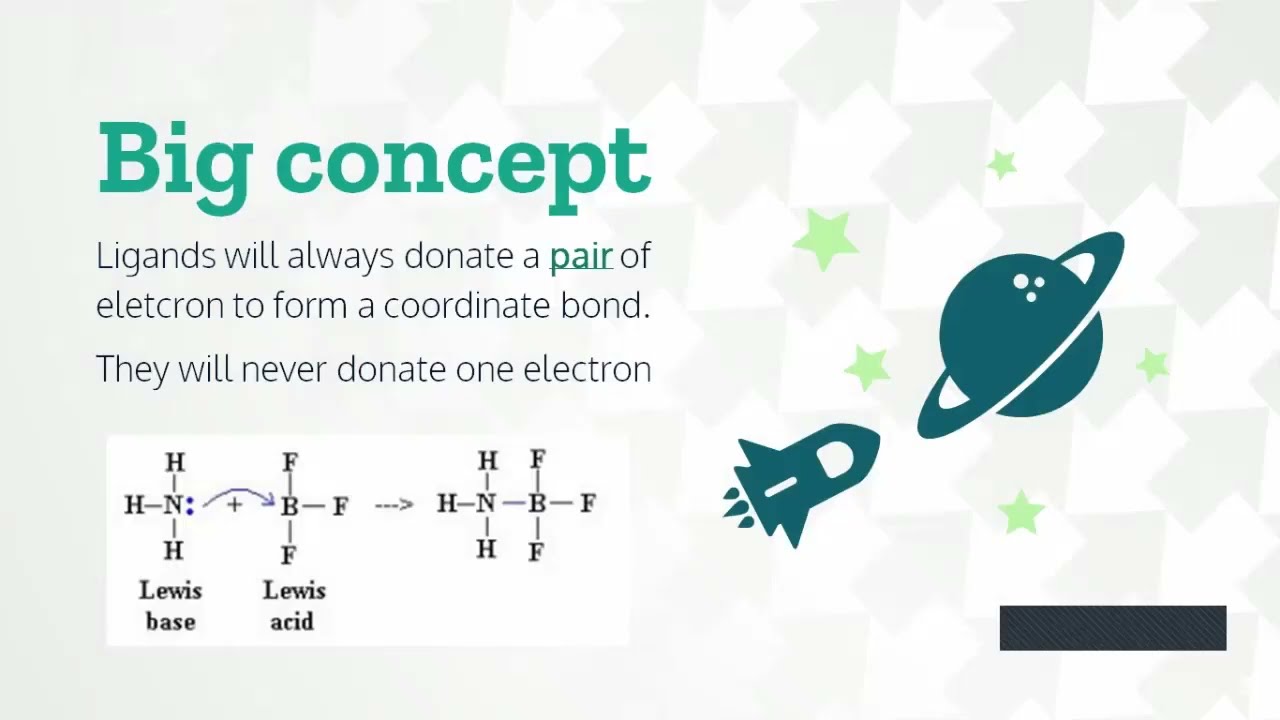
The concept of electron donating groups is best understood through examples. Let’s take a look at some common coordinate complexes formed by these groups:
Ferrocene
Ferrocene, with the chemical formula Fe(C5H5)2, is an organometallic compound composed of two cyclopentadienyl ligands bonded to a central iron atom. The cyclopentadienyl rings, being aromatic in nature, donate their pi-electrons to the metal center, thereby forming a stable coordination bond. Ferrocene finds extensive use in catalysis and organic synthesis due to its stability and reactivity.
Cobalt(III)-amine Complexes
Cobalt(III)-amine complexes, such as [Co(NH3)5Cl]2+, are widely studied in coordination chemistry. The ammonia ligands act as strong electron donors, forming coordinate bonds with the cobalt ion. These complexes exhibit a range of properties and have found applications in medicine, catalysis, and material science.
Grignard Reagents
Grignard reagents are organometallic compounds containing a carbon-magnesium bond. These reagents, formed by reacting an organic halide with magnesium metal, are powerful nucleophiles due to the electron donation from the magnesium atom. They are commonly used in organic synthesis and can also form coordinate complexes with transition metals.
3. Comparing Electron Donating and Withdrawing Groups

Electron donating groups can be contrasted with electron withdrawing groups, which have an opposite effect on the central atom’s electron density. While electron donating groups increase the electron density around the central atom, electron withdrawing groups decrease it by withdrawing electrons through inductive effects.
This difference in electronic properties leads to varying reactivity and stability of the resulting coordinate complexes. Electron withdrawing groups tend to form more stable complexes due to their ability to polarize the central atom, whereas electron donating groups result in more reactive complexes.
4. Tips for Utilizing Electron Donating Groups in Coordinate Complex Formation
To effectively use electron donating groups in coordinate complex formation, here are some tips to keep in mind:
- Choose the appropriate ligand: The choice of ligand is crucial in forming a stable coordinate complex. Consider the central atom’s size, charge, and required coordination number before selecting the ligand.
- Understand the electronic properties of the group: As discussed earlier, different types of electron donating groups have varying electronic properties and therefore contribute differently to complex formation. Understanding these differences can help in choosing the right group for a specific reaction.
- Consider steric effects: Large and bulky groups may not fit into the coordination sphere of the central atom, hindering complex formation. It’s essential to consider the steric effects of the group when designing a coordination complex.
FAQs
Q: Can a molecule have both electron donating and withdrawing groups?
A: Yes, a molecule can contain both types of groups. In such cases, the overall electron density around the central atom depends on the balance between the two groups. For example, in an organic molecule containing both a phenyl group (electron withdrawing) and an amino group (electron donating), the electron density around the central atom will be lower than that of the parent compound.
Q: Can electron donating groups form coordinate complexes with non-transition metal ions?
A: Yes, electron donating groups can form coordinate complexes with non-transition metal ions, such as alkali and alkaline earth metals. However, the resulting complexes may have different properties compared to those formed with transition metals.
Q: How does the strength of the coordinate bond vary with different electron donating groups?
A: The strength of the coordinate bond depends on the electron donating ability of the group. Stronger electron donors, such as phosphines and amines, form more stable bonds compared to weaker donors, such as alkyl groups.
Q: Are there any limitations to the use of electron donating groups in coordinate complex formation?
A: Some limitations include the steric hindrance of large groups and the potential for unwanted side reactions when using highly reactive electron donating groups.
Q: How does the choice of solvent influence the formation of coordinate complexes with electron donating groups?
A: The solvent can play a significant role in coordinating with both the ligand and the central atom, affecting the stability and reactivity of the complex. Non-polar solvents tend to favor the formation of more stable complexes, while polar solvents can enhance the reactivity of the complex.
Conclusion
In conclusion, electron donating groups are essential players in the formation of coordinate complexes. They donate their lone pairs of electrons to the central atom, stabilizing and influencing the reactivity of the complex. Their versatility and ability to form strong coordinate bonds make them valuable tools in synthetic chemistry. By understanding the various types and properties of these groups, we can utilize them effectively in designing and synthesizing new and complex molecules.
